Summary: Researchers have discovered a method for carefully targeting particular NMDA receptors in the mind to treat depression. Unlike high-dose anesthetic effects, low-dose morphine binds to longitudinal holes on NMDA receptors, enhancing excitable transmitting and long-term neural changes. This method virtually immediately relieves depressive symptoms and continues to help people even after ketamine has been metabolized.
The review provides a framework for creating likely safer, orally administered ketamine-like medications. Scientists may better understand despair and develop brain disorder treatments by identifying these bound sites.
Important Information:
- Low-dose morphine targets certain NMDA receptor sites, enhancing activating transmission.
- This result explains ketamine’s swift and long-lasting opioid impact.
- Discovering ketamine’s bound places may lead to safer, orally administered options.
Origin: University at Buffalo
Low-dose morphine has been identified as the binding page of a study by University at Buffalo, providing crucial insight into how the treatment, frequently referred to as a wonder drug, treats major depression signs in as little as a few hours with prolonged effects lasting several times.
The UB finding, which was published in September in Molecular Psychiatry, may also aid in the identification of how unhappiness comes from within the brain and may encourage further research into the use of ketamine and ketamine-like medications for other brain disorders.
A lifesaving medication
Ketamine has been used as an analgesic since the 1960s, but a much lower dose of it was tested in its first clinical trial in 2000 to demonstrate its rapid potency in treating significant depression and suicidal ideation.
” Due to its fast and long-lasting results, low-dose morphine proved to be actually a rescue medicine”, says , Gabriela K. Popescu, PhD, senior author on the study and professor of biology in the Jacobs School of Medicine and Biomedical Sciences at UB.
Traditional meds take months to start working, which increases the risk of some individuals having suicidal thoughts at the start of treatment. Cocaine works almost immediately to treat depression symptoms and is effective for a few days and up to a fortnight after administration.
Since this study was published in the early 2000s, morphine centers, where the medicine is administered orally to treat unhappiness, have been established in cities global.
But how ketamine has such a potent antidepressant effect so quickly has not been fully understood molecularly. This understanding is crucial for figuring out how to make ketamine and how to create other similar medications.
Selective effects on NMDA receptors
Ketamine binds to a class of neurotransmitter receptors called N-methyl-D-aspartate ( NMDA ) receptors. Popescu is an expert on how these receptors produce electrical signals that are essential for cognition, learning and memory, and how these signals, when dysregulated, result in psychiatric symptoms.  ,
In this article, Popescu explains how ketamine can be very low-dose NMDA receptors ‘ activity can be affected by only a small number of cases.
The brain contains NMDA receptors that are essential for maintaining consciousness. For this reason, she explains, drugs that act indiscriminately on all NMDA receptors have unacceptable psychiatric side effects.
According to Popescu,” we think the selectiveness we found in our research explains how low-dose ketamine can treat major depression and stop suicide in depressed people,”  ,
The research was sparked by an observation in her lab by co-author Sheila Gupta, then a UB undergraduate.
According to Popescu,” Sheila noticed that ketamine had a stronger inhibitory effect than anticipated when applied to chronically active NMDA receptors,” according to Popescu. ” We were curious about this discrepancy”.
Researchers tried to understand how ketamine’s antidepressant effects worked when it first became known by putting it on NMDA receptor-produced synaptic currents, but the drug had little to no effect.
” This observation caused many experts to turn their attention to receptors located outside synapses, which might be mediating ketamine’s antidepressive effects”, Popescu says.
We were interested in discovering other mechanisms besides the direct current block, which was assumed to be the only mechanism that ketamine had on NMDA receptors in response to Sheila’s observation that ketamine was a stronger inhibitor of receptors that are active for longer periods.
Few labs with this NMDA expertise
The NMDA receptors become active process is one of only a few in the world, thanks to Popescu’s lab. When ketamine was present at very low doses versus when it was present at high ( anesthetic ) doses, Popescu and her colleagues were able to identify and precisely determine what exactly changed during the NMDA activations.
We can track each receptor’s entire behavioral repertoire because we can track activity from a single receptor molecule over a long period of time, and we can identify which aspects of the process are altered when the receptor binds to a drug or carries a mutation, Popescu explains.
” The mechanism we uncovered suggests that at low doses, ketamine will only affect the current carried by receptors that had been active in the background for a while, but not by synaptic receptors, which experience only brief, intermittent activations”, she continues.
” This results in an immediate increase in excitatory transmission, which in turn lifts depressive symptoms. Additionally, the rise in excitation results in the formation of new or stronger synapses, which help maintain higher excitatory levels even after the body clears of ketamine, contributing to the long-term relief seen in patients.
The UB study provides an explanation for why such low ketamine dosages work.
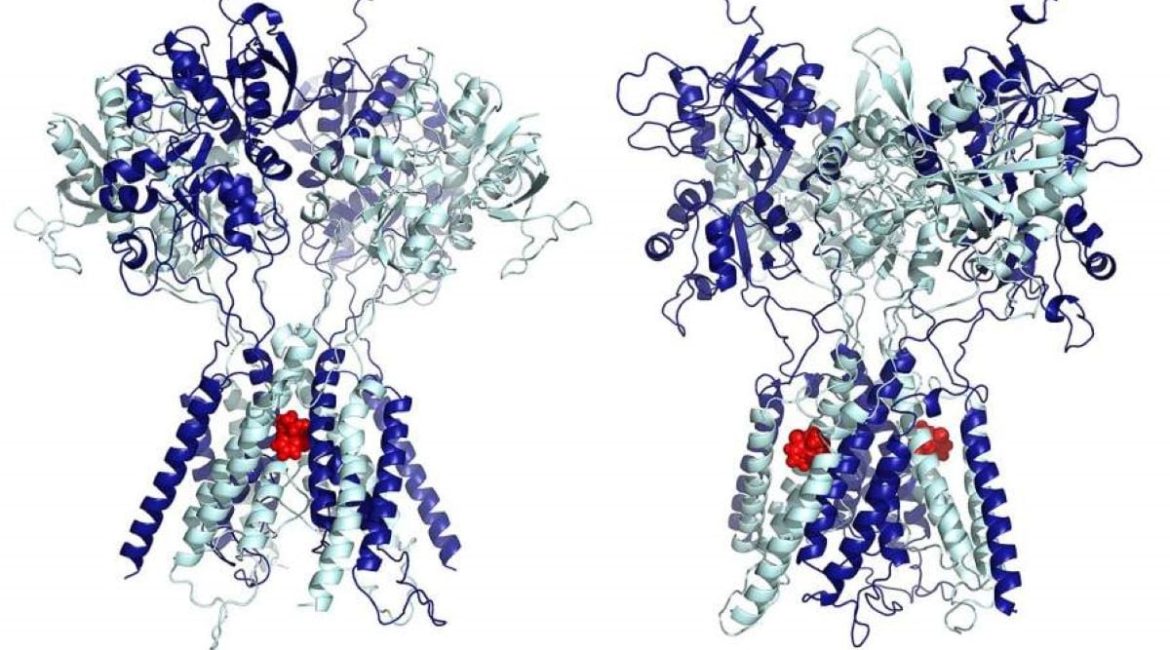
” Our results show that very low levels of ketamine, on the nanoscale, are sufficient to fill two lateral grooves of the NMDA receptors to selectively slow down extra-synaptic receptors, alleviating depression. The anesthetic effect is initiated when the dose is raised by increasing the amount of ketamine that spills from the grooves into the pore and begins to block synaptic currents. says Popescu.
The Department of Physics at the College of Arts and Sciences, along with Popescu, created a three-dimensional model of the NMDA receptor and identified the precise residues where ketamine binds at the lateral sites. She claims that these interactions are powerful and contribute to the receptor’s high ketamine affinity.
” The simulations show that at high concentrations, which is how it is used as an anesthetic, ketamine indeed lodges itself in the central ion-conducting pore of the receptors, where it stops ionic current from flowing through the receptor”, says Popescu.
In contrast, at low concentrations, ketamine functions very differently, attaching to two symmetrical sites on the sides of the pore, such that instead of stopping the current, ketamine makes receptors slower to open, reducing the current only a little bit.
The ideal template for creating ketamine-like drugs that can be administered orally and that may not have the addictive potential of ketamine is found in finding the exact binding site on the receptor, Popescu says.
The best course of action is to first computationally and then experimentally screen existing medications for fitment into the lateral grooves of NMDA receptors.  ,
Lead authors are Jamie A. Abbott, PhD, in the Department of Biochemistry, and Han Wen in the Department of Physics. Other co-authors are Gupta, Wenjun Zheng Beiying Liu and Gary J. Iacobucci.
Funding: The research was funded by the National Institutes of Health.
About this information about research in depression and psychopharmacology
Author: Ellen Goldbaum
Source: University at Buffalo
Contact: Ellen Goldbaum – University at Buffalo
Image: The image is credited to Jamie Abbott
Original Research: Closed access.
Jamie Abbott and colleagues ‘ work on” Low Dose Ketone: Allosteric Inhibition of NMDA Receptors.” Molecular Psychiatry
Abstract
Low dose ketamine can inhibit the NMDA receptors allosterically.
Ketamine, a general anesthetic, has rapid and sustained antidepressant effects when administered at lower doses. By binding deeply into the NMDA receptor pore, where it blocks current influx, ketamine atnesthetic levels lower excitatory transmission.
In contrast, the molecular targets responsible for antidepressant levels of ketamine remain controversial.
We used electrophysiology, structure-based mutagenesis, and molecular and kinetic modeling to investigate the effects of ketamine on NMDA receptors across an extended range of concentrations.
We report functional and structural evidence that, at nanomolar concentrations, ketamine interacts with membrane-accessible hydrophobic sites on NMDA receptors, which are distinct from the established pore-blocking site.
These interactions stabilize receptors in pre-open states and produce an incomplete, voltage- and pH-dependent reduction in receptor gating.
Notably, this allosteric inhibitory mechanism avoids brief synaptic-like receptor activations and preferentially reduces currents from neurotransmitters-activated receptors.
We suggest that the hydrophobic regions we describe here play a role in the clinical outcomes of ketamine, which are not shared by memantine and other open-channel NMDA receptor blockers, and that they make promising candidates for the development of potent, potent, and potent neuroactive treatments.