Summary: Scientists have discovered a revolutionary tool known as Helicase-Assisted Continuous Editing ( HACE), which allows them to create precise genetic modifications for particular genes without altering the rest of the genome. HACE improves our ability to study protein functions and disease systems by combining helicase enzymes and CRISPR systems with precise Genome sequences. The tool’s possible for medical discovery has been demonstrated by its ability to identify drug resistance mutations in cancer-related chromosomes and cutting defects in blood tumors.
Important Facts:
- HACE introduces mutations in particular genes, leaving the rest of the chromosome untouched.
- It has identified mutagenicities that are linked to cloning problems and cancer drug resistance.
- This tool may revolutionize genetic and healing research.
Origin: Harvard
Good and bad effects can be caused by protein mutations, from insulin resistance to sensitivity to certain cancers.
Scientists must immediately infect human cells to examine these mutations. However, it takes a difficult to modify the biological blueprints inside of cells. 3 billion basic groups of DNA are distributed across tens of thousands of chromosomes, making up the human genome.
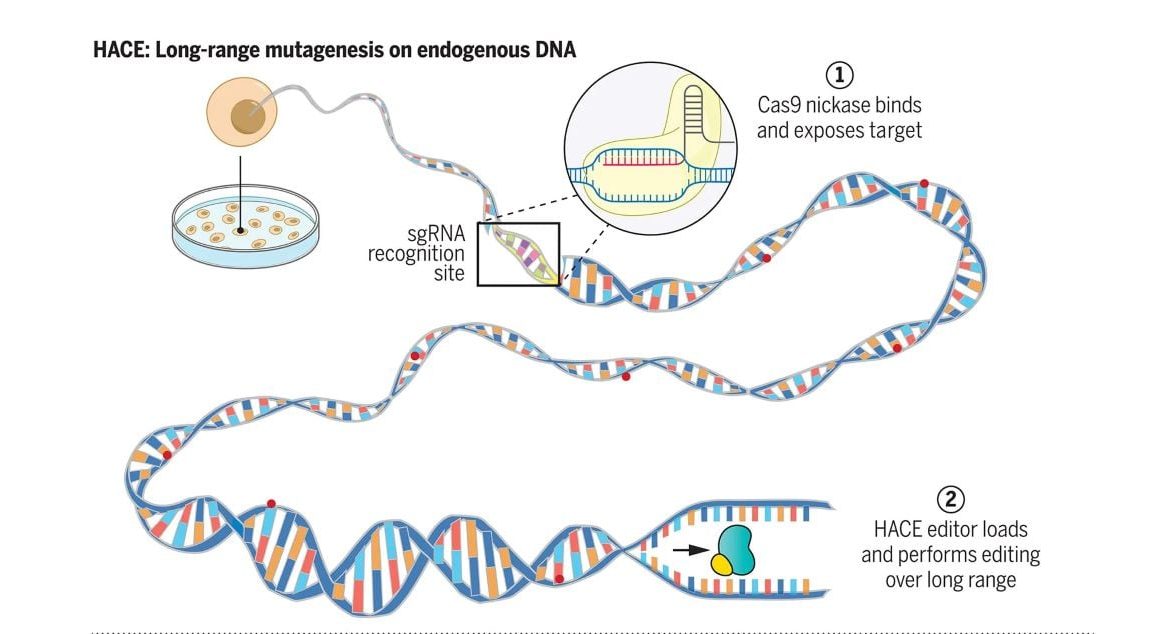
To accomplish this, Harvard researchers have developed a device that makes it possible to quickly change specific chromosomes in the interest without altering the rest of the genome. Described in , Science, their tool, called Helicase-Assisted Continuous Editing ( HACE), can be deployed to predetermined regions of the genome in intact, living cells.
A Griffin Graduate School of Arts and Sciences scholar studying synthetic biology in the Department of Stem Cell and Regenerative Biology, Xi Dawn Chen, the first artist, described the development of tools like this as a major step forward in our ability to harness development straight within human cells.
This device allows for precise mutagenesis in particular regions of the genome, allowing the development of enzymes and treatments previously untapped.
HACE has the advantage of being directed to specific areas rather than just a town, in contrast to existing methods for mutagenesis, which involve inserting additional copies of genes or loosely mutating multiple different genes at once. The group’s novel nanotechnology involves combining a helicase, which is an enzyme that normally “unzips” DNA, with a gene-editing protein.
They then use the gene-editing systems CRISPR-Cas9 to guide the proteins piece to the gene they want to change. Just that protein series is affected by abnormalities as the helicase unzips the DNA.
Older author Fei Chen, associate professor in the Department of Stem Cell and Regenerative Biology and part of the , Broad Institute, described HACE as a powerful instrument for targeted development because of its combination of CRISPR’s precision and ability to modify lengthy stretches of DNA.
The scientists used the tool to identify drug resistance mutations in a gene called MEK1 to demonstrate its utility in the lab. Cancer treatments frequently target this gene but frequently fail because the diseased cells mutate resistance mechanisms.
The team used HACE to identify several distinct changes that are related to cancer drug resistance, including selumetinib and trametinib, giving insights into how mutations affect drug performance.
They also examined how mutations in SF3B1, a gene involved in a biomolecular process called RNA splicing, affects RNA assembly. Mutations in this gene are common in blood cancers, but it’s been unclear which mutations cause the splicing defects, with HACE, the team could easily identify those changes.
The researchers also used the tool to better understand how changes to a regulatory DNA region affect the production of a protein in immune cells that may be a potential target for cancer immunotherapies, in collaboration with , Bradley Bernstein’s lab , at Harvard Medical School, and Dana-Farber Cancer Institute.
According to Bernstein, HACE and other tools might one day enable significant editing of gene regulatory sequences, which could be combined with deep learning computation for deciphering. According to Bernstein, “one can imagine many new therapeutic opportunities that involve precise editing or tuning of these regulatory sequences to “fix” gene activity and lessen disease.”
Funding: This research was supported by multiple sources including the National Institutes of Health, the Broad Institute, and the Harvard Stem Cell Institute.
About this news from genetics research
Author: Anne J. Manning
Source: Harvard
Contact: Anne J. Manning – Harvard
Image: The image is credited to Neuroscience News
Original Research: Closed access.
” Endogenous genomes can be programmed to mutagenesis using helicase-assisted continuous editing.” by Xi Dawn Chen et al. Science
Abstract
Endogenous genomes can be programmed to mutagenesis using helicase-assisted continuous editing.
INTRODUCTION
The three billion bases of the human genome have an enormous impact on protein function and gene regulation, which is a fundamental challenge for genomics. Therefore, it is crucial to develop strategies for systematic and high-throughput mutagenization of genomic sequences.
In particular, targeted mutagenesis of single genomic loci could emulate the natural evolution process to reveal sequence-structure relationships, gain- and loss-of-function phenotypes, and cooperative mutations. No technique is currently available to carry out continuous mutagenesis at specific locations in mammalian cell endogenous genomes.
RATIONALE
We developed a tool to perform endogenous mammalian genome-targeted mutagenesis. We discovered that helicases are powerful processors that can travel large genomic areas in nature. Some helicases, including those involved in DNA damage repair, can load and start unwinding DNA at single-stranded DNA regions in the genome.
When fused to a deaminase enzyme, we argued that these helicases could be used for long-range, targeted mutagenesis. The fusion construct and its interval of hypermutation could then be programmably targeted, through single-guide RNAs (sgRNAs ), to specific genomic regions using a Cas9 nickase. Random mutations will then be produced in the region as a result of the recruited helicase’s directional and long-range DNA unwinding event.
RESULTS
We created a platform called helicase-assisted continuous editing ( HACE), which combines CRISPR gene editing tools ‘ long-range sequencing and sequence programmability. HACE uses CRISPR-Cas9 to direct the loading of a helicase-deaminase fusion for targeted hypermutation of the downstream genomic sequence. HACE was able to decode locus-specifically across 1000 nucleotides that had been continuously accumulating over the course of 1000 years.
We further evaluated HACE prototypes incorporating diverse helicases, Cas9 variants, and deaminases, showing that they have tunable edit rates and ranges. We also demonstrated that the guide RNAs used in this study can be multiplexed to target multiple genomic regions at the same time. Then, using HACE, we functionally examine endogenous mutations that cause enzymatic change, cis-regulatory element function changes, and enzymatic activity changes in both coding and noncoding genomic contexts.
In the coding space, we identified variants that lead to mitogen-activated protein kinase kinase 1 ( MEK1 ) –inhibitor drug resistance and also identified variants in SF3B1, a splicing factor, that lead to alternative 3′ splice-site usage. We identified functional artificial variants in CD69’s enhancer regions and identified specific bases and motifs that influence the regulation of CD69 in specific bases and motifs.
HACE addresses the challenges that conventional base editing screens face: the need for an NGG protospacer adjacent motif in the sgRNA recognition sequence and the presence of bystander mutations that can lead to artificial linkages and tainted screening results. HACE’s extensive editing range allows for combinatorial effects and interactions between multiple distant mutations at the same locus.
CONCLUSION
HACE makes possible the continuous, long-range, programmable diversification of endogenous mammalian genomes. We anticipate that HACE will significantly expand the functional genomics toolbox and enable the creation of systematic sequence-function maps of both coding and noncoding genomes.
Additionally, the endogenous genome can be transformed into a directed evolution system, allowing for the selection of sequences for desired mammalian functions.