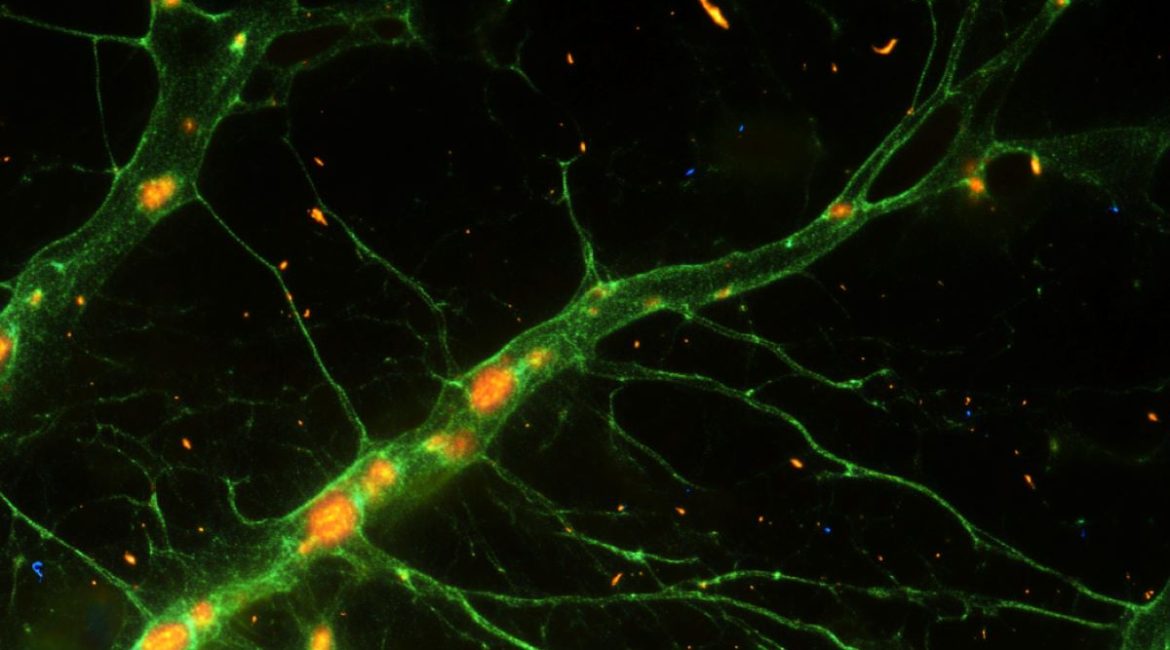
Summary: Scientists have effectively reprogrammed astroglia, a type of mental support battery, into neurons that mimic certain interneurons essential for brain work. They increased the Ascl1 protein’s ability to turn astroglia into neuron-like cells, opening up new avenues for restorative treatments for mental conditions like epilepsy.
High-frequency fire, a sign of some interneurons crucial to controlling mental activity, is observed in the engineered neurons. This study suggests that we could recover lost or damaged brain circuits by using astroglia as a repair tool.
Important Information:
- Astroglia are transformed into practical cells by the modified Ascl1 protein.
- Reprogrammed cells exhibit high-frequency fire, important for head circuit control.
- This method has the ability to restore neural circuits in situations like seizures.
Origin: King’s College London
Researchers have successfully demonstrated how to reprogramme astroglia, which are mind tissues that support mental function, into cells resemble interni.
The study, which was published in Science Advances, is both a significant advance in cerebral engineering and has important implications for renewable medicine, which researchers hope will be applied to repair brain circuits that are dysfunctional like those seen in epilepsy patients.
Scientists coaxed astroglia to produce a proteins, Ascl1, which is crucial for the development of the nervous system by working with animals shortly after birth.
They discovered that Ascl1’s mutation significantly increased its ability to transform astroglia into functioning cells, as opposed to the protein’s natural form, which the body produces.
We’re excited to display that engineered neurons may get very specific attributes, even though the neurons we induced are different from those the brain produces itself.
Our findings will help us close the gap between endogenous and prompted neurons, making them even more important for renewable medicine’s future translation. ” Said Professor Benedikt Berninger, Professor of Developmental Neurobiology at King’s IoPPN and the study’s senior writer
The research team discovered that the cells they created had characteristics that were similar to those found in the hippocampus they were developing, including the ability to fire very high frequencies, a telltale sign of a certain group of cells that are crucial for controlling brain circuitry.
One of the study’s lead writers, Dr. Nicolás Marichal, said,” This groundbreaking study pioneers new ground in restorative treatments, offering promises for the restoration of abnormal brain function and wiring in neurological conditions.
This job opens the door to more studies to use genealogy reprogramming of glia as a novel therapeutic strategy to utilise these findings.
Funding: This research was funded in part by Wellcome Trust, the European Research Council ( ERC ) under the European Union’s Horizon 2020 Research and Innovation Programme, and the German Research Foundation.
About this information from science study
Author: Benedikt Berninger
Source: King’s College London
Contact: Benedikt Berninger – King’s College London
Image: The image is credited to Neuroscience News
Original Research: Start exposure.
” Reprogramming astroglia into cells with marks of fast-spiking parvalbumin-positive neurons by phospho-site–deficient Ascl1″ by Benedikt Berninger et cetera. Science Developments
Abstract
Reprogramming astroglia into cells with marks of fast-spiking parvalbumin-positive neurons by phospho-site–deficient Ascl1
Mammalian glia may be reprogrammed to have cerebral fate through mobile reprogramming, which has the potential to restore damaged brain circuits.
While the proneural factor , achaete-scute complex-like 1 , ( Ascl1 ) is widely used for neuronal reprogramming, in the early postnatal mouse cortex,  , Ascl1 , fails to induce the glia-to-neuron conversion, instead promoting the proliferation of oligodendrocyte progenitor cells ( OPC ).
Since Ascl1 activity is posttranslationally regulated, here, we investigated the consequences of mutating six serine phospho-acceptor sites to alanine ( Ascl1SA6 ) on lineage reprogramming in vivo.
Ascl1SA6 exhibited increased neurogenic activity in the glia of the early postnatal mouse cortex, an effect enhanced by coexpression of , B cell lymphoma 2 , ( Bcl2 ).
Most induced cells were created by astrocytes, while only a small number were created by OPCs, according to biological fate-mapping.
Some Ascl1SA6/Bcl2-induced neurons expressed parvalbumin and were worthy of high-frequency action possible fire.
Our study expands our understanding of brain cerebral fates by demonstrating the real conversion of astroglia into neurons with subclass hallmarks of cerebral interneurons.