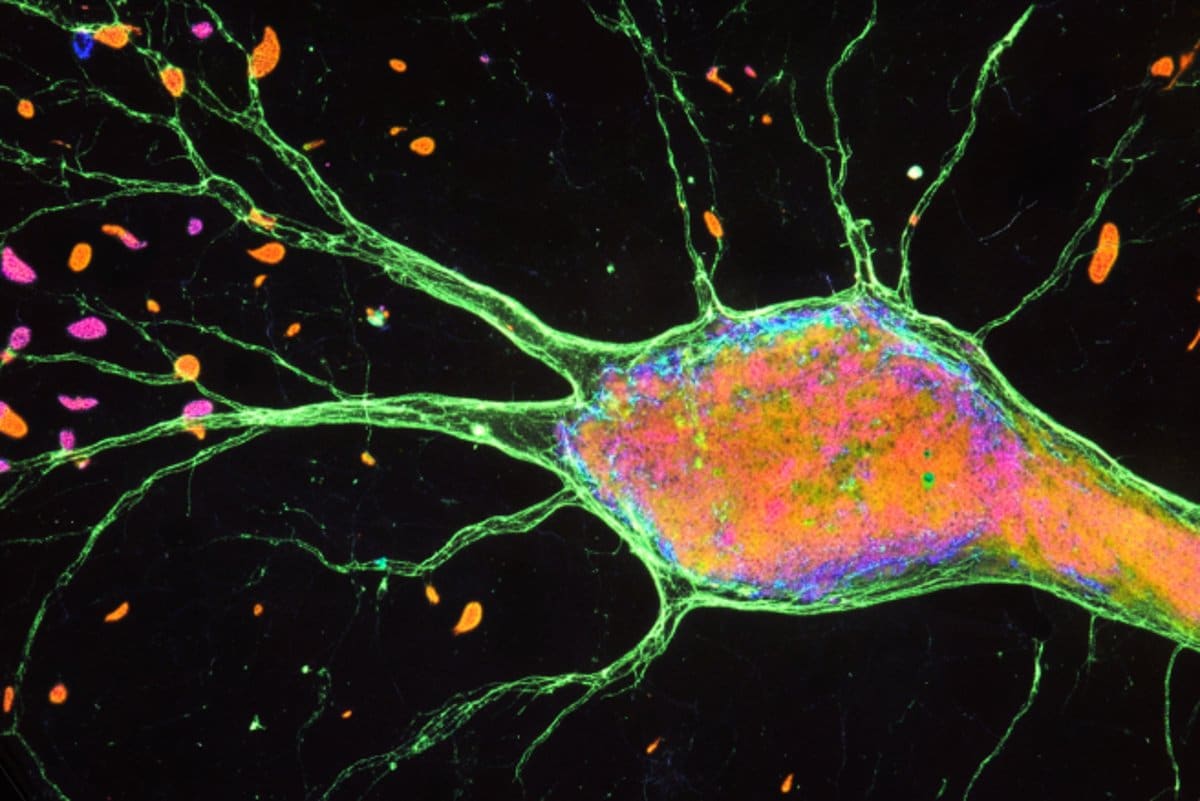
In a lab dish, researchers have used organoids to design the crucial brain and spinal cord regions that are responsible for delivering pain. This discovery allows scientists to see how discomfort signals travel from external neurons to the mind for the first time outside the system.
The concept, known as an assembloid, responds to signals that cause problems and reflects the effects of genetic mutations known to affect problems understanding. This development may improve drug discovery for pain relief, particularly for conditions like chronic problems or sensitivity.
Important Information:
- Full Pain Pathway Recreated: Four linked head and spinal organoids simulate people problems signal transmission.
- Drug Discovery Platform: The assembloid enables the assessment of possible pain-blocking and pain-inducing substances.
- Gene-Specific Perspectives: Mutations in Nav1.7 calcium stations altered wave-like neurological activity, replicating pain disorders.
Stanford, Stanford
Stanford Medicine prosecutors have replicated, in a laboratory food, one of humans ‘ most important nerve pathways for observing problems. This nerve circuit sends bodily sensations to the brain via the skin.
Once further processed in the brain, these signals will translate into our subjective experience, including the uncomfortable feeling of pain.
The advancement promises to accelerate what has been slow progress in understanding how pain signals are processed in people and how to minimize pain.
In a study to be published April 9 in , Nature, scientists led by , Sergiu Pasca, MD, the Kenneth T. Norris, Jr., Professor II of Psychiatry and Behavioral Sciences, describe their successful assembly of four miniaturized parts of the human nervous system to reconstitute what’s known as the ascending sensory pathway.
The dorsal root ganglion, the dorsal spinal cord, the thalamus, and the somatosensory cortex are the four distinct regions of the ascending sensory pathway that are connected to the brain by nerve cells, or neurons.
” We can now model this pathway non-invasively”, said Pasca, the study’s senior author. We hope that will lead to better understanding of how to treat pain disorders.
Lead co-authors of the study are postdoctoral scholars Ji-il Kim, PhD, and Kent Imaizumi, MD, PhD.
According to Pasca, who is also the Bonnie Uytengsu and the Stanford Brain Organogenesis Program’s Family Director, human pain has frequently proved challenging to study in laboratory animals. ” Their pain pathways are in some respects different from ours”, he said. ” These animals also experience pain. Our dish-based construct doesn’t”.
No one has been able to watch the information that is being transmitted across this entire pathway as of yet.
But Pasca and his colleagues witnessed never-before-seen waves of electrical activity travel from the first component of their construct all the way to the last, and they were able to enhance or disrupt these wavelike patterns by gene alterations or chemical stimulation of elements of the circuit.
Better medications were required
” Pain is a huge health problem”, said , Vivianne Tawfik, MD, PhD, associate professor of anesthesiology, perioperative and pain medicine, who was not involved in the study.
More than one in three Americans experience some form of chronic pain, according to her statement.” Some 116 million Americans are affected by one type or another of chronic pain.” This pain often persists even when observable damage is no longer evident, possibly due to lasting changes in the ascending sensory pathway.
She said,” I can’t even tell you how sad it is to sit in front of a patient who’s suffering from chronic pain after we’ve tried everything and there’s nothing left in our arsenal,” but treatments for chronic pain are scarce and far from ideal.
Most “pain medications” aren’t licensed for pain per se but instead borrowed from psychiatrists ‘ or sleep-disorder specialists ‘ medicine cabinets.
The most potent painkillers in the group are opioids, which have the unfortunate side effect of making people who have chronic pain habit-forming and make them more susceptible to addiction.
Building the sensory pathway piece by piece
Tawfik said she believes the team’s new construction is extremely relevant for the study of chronic pain.
” The pathway they’ve reconstructed is the most important one for conveying pain-related information”, she said.
Three sets of neuronal connections connect the regions that make up the ascending sensory pathway: the first set relays sensory information from the skin to the dorsal root ganglion to the spinal cord, the second set of neurons transmits the signals to a brain-called thalamus, and the third relays this information from the thalamus to the somatosensory cortex for processing the sensory information coming from the periphery.
Pasca has pioneered the creation of what he calls regionalized neural organoids, grown in a lab dish from stem cells and representing various distinct brain regions.
Pasca has recently developed this technology by combining organoids of different types in a dish so they can form what he calls assembloids.
Neurons from one organoid, via growth or migration, can penetrate the other organoid to form working circuits functionally similar to, or even identical to, those they’re meant to mimic.
As long as we make the parts and put them together correctly, we’ve been finding that we don’t need to know the details of assembly of these circuits, Pasca said.
” Once you put the organoids together, the cells find each other and connect in a meaningful way, giving rise to new features”.
In the most recent study, Pasca and his colleagues combined four of the sensory pathway’s four key regions into a human organoid that resembles the pathway by combining them together in series to create an assembloid.
Starting with cells from skin samples from volunteers, the team first transformed them into induced pluripotent stem cells, which are essentially de-differentiated cells that can be guided to become virtually any cell type in the human body.
Each of the four regions of the pathway is represented by tiny balls called neural organoids, which the researchers used to coax these cells into tiny balls called neural organoids.
Each organoid was a bit less than 1/10 inch in diameter and contained close to a million cells.
Pasca and his colleagues waited by placing the organoids of those four different types side by side. About 100 days later, they had fused into an assembloid almost 2/5 of an inch long —” They look like tiny sausage links”, Pasca said — and consisting of nearly 4 million cells.
That’s less than the 1 / / / / / / / / / / 1 / / 1 / / 1 / 1 / 1 / 1 / 1 / 1 / 1 / 1 / 1 / 2 of the human brain, which contains about 170 billion cells, Pasca noted. But the construct recapitulated the circuitry involved in the pathway.
The researchers demonstrated that the constituent organoids of the assembloids were anatomically related: neurons from the first had worked connections with neurons from the second, the second with the third, and so on.
Moreover, the entire circuit, from the sensory organoid to the cortical organoid, worked as a unit. Patterns of spontaneous, synchronized, directional signaling within the assembloid emerged once all four organoids had been sat next to each other in the flask for about 100 days: neuronal activity in the sensory organoid, thalamic organoid, and finally cortical organoid.
” You’d never have been able to see this wavelike synchrony if you couldn’t watch all four organoids, connected, simultaneously”, Pasca said. The brain is more than the parts ‘ parts combined.
Hot peppers and sodium channels
The wavelike activity in the assembloids was increased by chemicals known to cause pain. Stimulating the sensory organoid with capsaicin — the ingredient in chili peppers that produces a burning sensation in our mouths — triggered immediate waves of neuronal activity.
Rare genetic changes to an ion-exchange protein found on the surfaces of peripheral sensory neurons can cause crippling hypersensitivity to pain or, conversely, a potentially life-threatening inability to experience pain, significantly escalating the physical dangers that life presents, routine or otherwise.
The protein in question, Nav1.7, is a particular type of sodium channel that, the researchers observed, abounds on peripheral sensory neurons but is scarce elsewhere. The scientists created an assembloid in which the original sensory component’s normal version of Nav1.7 was replaced by the mutant pain-hypersensitivity version.
The resulting mutant sensory assembloids displayed more-frequent waves of spontaneous nervous transmission from the sensory through the spinal and thalamic organoids to the cerebral-cortex organoid.
Instead of rendering the same sodium channel in the sensory organoid non-functional, Pasca’s team discovered a surprise: Firing from that organoid in response to a pain-inducing chemical continued, but the synchronized wavelike transmission of pain information through the circuit mysteriously vanished. The firing was now out of sync.
Pasca claimed that” the sensory neurons still fired.” ” But they failed to engage the rest of the network in a coordinated manner”.
Importantly, organoids representing other brain regions that are crucial to the discomfort experienced by those who are in pain were absent from these assembloids.
” The assembloids themselves don’t’ feel’ any pain”, Pasca said.
They” transmit nervous signals that need to be further processed by other brain regions for us to experience the unpleasant, disorienting feeling of pain.”
The assembloids, after only a few months of their assembly, represent an early phase of fetal development, he said. Their immediate application would be in studying autism and other neurodevelopmental disorders.
People with autism are often hypersensitive to pain and to sensory stimulation in general, and some autism-associated genes are active in the sensory neurons of the ascending sensory pathway.
Pasca claimed that his lab is developing methods to better understand how the pathway they represent functions in adults and whether or not it does.
” Nav1.7 appears to exist mostly on the surfaces of peripheral pain-sensing neurons”, Pasca said.
We believe that screening for drugs that tame sensory organoids ‘ ability to trigger excessive or inappropriate waves of neuronal transmission through our assembloid without affecting the brain’s reward circuitry as opioid drugs do — which is why they’re addictive — could lead to better-targeted treatments for pain.
Stanford University’s office of technology licensing has filed a patent for intellectual property associated with this assembloid, with Pasca, Kim and Imaizumi named as co-inventors.
The University of North Carolina researcher made a contribution to the study.
Funding: The study was funded by the National Institutes of Health ( grants R01MH107800, R01NS128028 and CNCDP-K12 ), the NYSCF Robertson Stem Cell Society, the Stanford Human Brain Organogenesis Project, the Kwan Research Fund, the Senkut Research Funds and the Chan Zuckerberg Initiative.
About this news about neurotech research and pain
Author: Bruce Goldman
Source: Stanford
Contact: Bruce Goldman – Stanford
Image: The image is credited to Neuroscience News
Original Research: Open access.
Sergiu Pasca and colleagues ‘” Human model of the ascending neural sensory pathway.” Nature
Abstract
Human model of the ascending neural sensory pathway
Somatosensory pathways convey crucial information about pain, touch, itch and body part movement from peripheral organs to the central nervous system.
Clinical translation continues to be challenging despite significant research still required to understand how these pathways come together and how to create and develop painkillers. This is probably related to species-specific features and the lack of in vitro models of the polysynaptic pathway.
In order to model the spinothalamic pathway, we developed a human ascending somatosensory assembloid ( hASA ), a four-part assembloid created from human pluripotent stem cells that incorporates somatosensory, spinal, thalamic, and cortical organoids.
Transcriptomic profiling confirmed the presence of key cell types of this circuit. Rasidiol tracing and calcium imaging demonstrated that sensory neurons communicate with dorsal spinal cord neurons, which then communicate with thalamic neurons.
Following noxious chemical stimulation, calcium imaging of hASA demonstrated a coordinated response. Additionally, synchronized activity was found throughout the assembly through extracellular recordings and imaging.
Notably, loss of the sodium channel NaV1.7, which causes pain insensitivity, disrupted synchrony across hASA.
In contrast, the gain-of-function, SCN9A, variant, is linked to extreme pain disorder-induced hypersynchrony.
These experiments demonstrated the ability to functionally assemble the essential components of the human sensory pathway, which could accelerate our understanding of sensory circuits and facilitate therapeutic development.