Summary: New research has revealed that Alzheimer’s disease may affect brain function by altering the proteins that form at the crucial interface between neurons and their myelin sheath. Researchers discovered structural anomalies in the paranode area, which includes clogged mineral stations and protein buildup that may prevent transmission transmission, in the region where myelin attaches to axons.
Amazingly, full myelin levels appeared to be unchanged, which suggests that dysfunction exists at a microscopic level. These findings highlight earlier unexplored targets for intervention and provide new information on how axonal signaling functions in Alzheimer’s.
Important Information:
- Disrupted Paranodes: Alzheimer’s blocks electronic indicating by altering proteins at the axon-myelin bridge.
- Amyloid Buildup: Spiral-shaped amyloid payments may block nutritional programs and lead to axonal swelling.
- Myelin Intact: Myelin’s architectural function is questioned, but ultimately myelin quantity is stable.
Origin: Yale
Alzheimer’s disease is related to the disruption of axons, the thread-like portion of brain tissue that transmit electrical signals. Harm to the myelin sheath, a greasy covering that covers axons, may be one way to hinder axonal functionality.
The nerve sheath allows cells to communicate with each other immediately, much like the plastic or rubber used to protect a wire. The transmission of electrical signals also suffers when the construction is compromised.
Researchers at Yale studied molecules in human brain cells, focusing on the sub-compartment that separates an neurons from its myelin sheath, to better understand any pathological procedures related to Alzheimer’s disease that may influence the myelin sheath.
They discovered structural abnormalities at the myelin-axon software that may impair electric signaling and that this sub-compartment had proteins that were different between those who had Alzheimer’s disease and those who did not.
The team’s findings were published in Nature Science on June 13th.
We might be able to identify the causes of disease development by learning how the proteins that make up the myelin sheath differ in the diseased state from the non-diseased state, says the study’s principal investigator, Jaime Grutzendler, MD, Dr. Harry M. Zimmerman, and Dr. Nicholas and Viola Spinelli, Professor of Neurology and Neuroscience at Yale School of Medicine ( YSM).
Different proteins compositions are present in Alzheimer’s condition.
Different cell types are affected by Alzheimer’s disease in different ways, and neurones are particularly susceptible to the condition. These cell produce the nerve that surrounds and defends neurons. One of the reasons the myelin sheath is fascinating to study is because of this.
The research scientist who spearheaded these studies, first author Yifei Cai, PhD, who served as the lead author of these studies, was part of Grutzendler’s team, who used a method that used a particular antibody to tag every protein in their area of interest. That made it possible for the researchers to identify the protein using mass spectroscopy after identifying them.
According to Grutzendler,” This method allows us to examine the specific protein that are contained within the pretty, very small area of the nerve sheath.”
Their assessments revealed proteins differences between good and wholesome tissue in Alzheimer’s disease. Some of these variations were related to fat metabolism, axon growth, and the formation of amyloid, an abnormal protein aggregate that accumulates in tissues and is linked to Alzheimer’s disease.
According to Grutzendler,” Myelin requires a lot of fat [a group of molecules that includes oils ] for regular work.”
” In Alzheimer’s condition, lipid metabolism may become abnormally affected in a way that alters myelin’s regular work.”
Imaging studies reveal anomalies in the paranode area.
The group also analyzed brain tissue samples using a super-resolution scanning technique known as expansion microscopy. Ironically, they discovered that age-matched good controls did not exhibit significant differences in the amount of nerve in the tissues affected by Alzheimer’s illness.
According to Grutzendler,” It seems that the entire amount of the nerve in the axons sheaths is fairly preserved.”
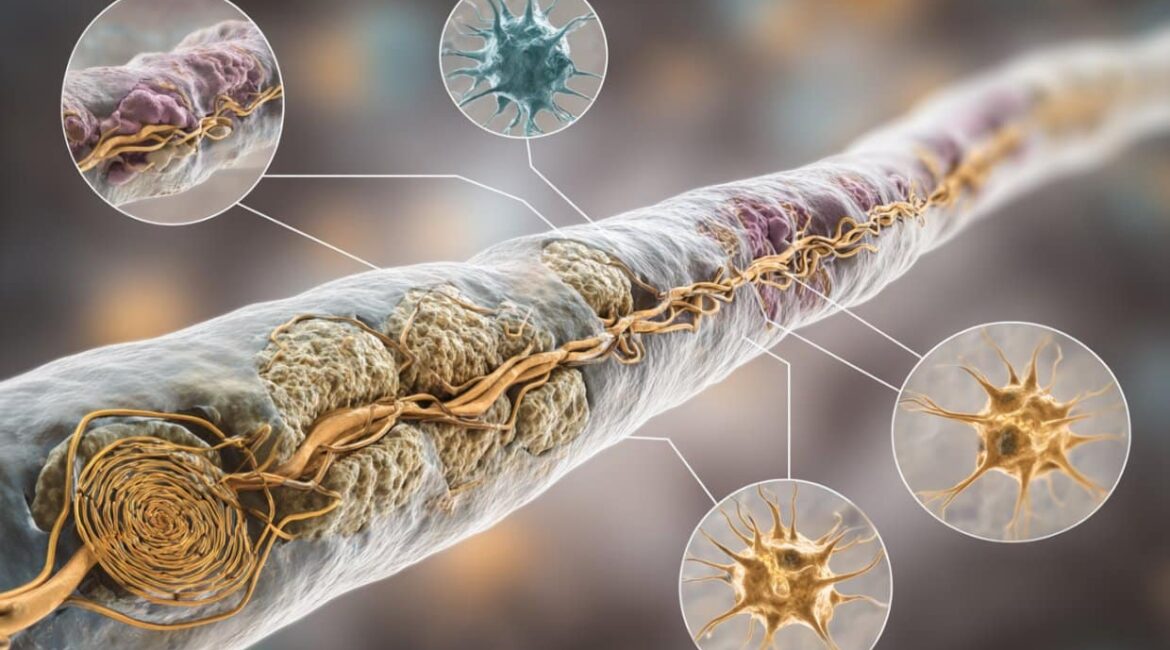
Emotions are covered in nerve, but there are small gaps called “nodes of Ranvier” where the brain is exposed to raise signs. Paranodes, which connect the myelin securely to the brain and aid in its anchoring and organization, are located just a few feet away from these gaps.
The team did not find any changes in the molecules at these paranodes, but they did, however, discover changes in the number of nerve. How well the brain impulses travel may be affected by these modifications.
Paranodes are also significant because they contain stations that help to move nutrition between the nerve and the nerve as well as distinct waste. The group discovered that amyloid may develop in distinctive spiral-shaped rings around the neurons, frequently forming close to the paranodes.
According to Grutzendler,” the formation of these amyloid protein has clogged these channels.” We believe that this is having an impact on the performance of the axon and lipid up as a result.
We might be able to identify what’s happening when the disorder develops if we learn how the molecules that make up the nerve sheath are affected in the damaged condition compared to a non-diseased condition, according to Jaime Grutzendler, MD.
In some cases, the scientists found axon swelling close to these amyloid rings. According to Grutzendler,” It’s probable that this amyloid formation around the neuron causes compression of the paranode channels to constrict and cause swelling.”
” It’s almost like tying a tie around a straw; if you constrict it and continue blowing into it, you’ll discover an extension of the grass by the knot.”
Additionally, myelin around  , axonal spheroids, which are bubble-like structures on neurons that form as a result of swelling, had abnormal designs.
Because of the spheroid’s impact on electrical transmission and the spheroidal’s various degrees of myelination, Grutzendler says,” This can have significant implications.” It’s” a triple hammer,” he says.
The staff at Grutzendler’s hopes to use these proteins data to identify areas where some of the abnormalities at the myelin-axon program can be improved.
According to Grutzendler,” We are still in the hypothesis-generating phase.” We still have a ton to do in the near future.
The study’s co-authors include Yale’s Fuyi Chen, Tram Huynh, Jean Kanyo, Peiyang Tang, Lukas Fuentes, Amber Braker, Rachel Welch, Lei Tong, Peng Yuan, TuKiet Lam, and Angus Nairn, Iguaracy Pinheiro-de-Sousa and Evangelia Petsalaki, and Mykhaylo Slobodyanyuk and Jüri Re
Funding: Yale University, the National Institutes of Health, and the National Institutes of Health ( awards RF1AG058257, R01NS115544, and R01NS111961 ) supported the research that was reported in this news article. The writers are only responsible for the content, which does not necessarily reflect the National Institutes of Health’s official position.
The research received funding from the Alzheimer’s Disease Research Center, Yale/NIDA Neuroproteomics Center Pilot Project Grant, BrightFocus Foundation, Yale Alzheimer’s Disease Research Center, Alzheimer’s Association, and EMBL Corporate Partnership Programme.
About this study on Alzheimer’s disease
Author: Isabella Backman
Source: Yale
Contact: Isabella Backman – Yale
Image: The image is credited to Neuroscience News
Initial studies has been made private.
Jaime Grutzendler et cetera.,” Myelin–axon program risk in Alzheimer’s illness revealed by subcellular proteome and scanning of human and mouse mind.” Science of the natural world
Abstract
Subcellular proteomics and brain imaging revealed a myelin–axon program risk in Alzheimer’s disease in the human and mouse brains.
For quick axonal conduction, physiological support, and cerebral plasticity, axons ensheathment is necessary.
Although the mechanisms underlying Alzheimer’s disease ( AD), myelin and axonal structures are affected, their causes are unknown.
In postmortem human brains from Advertising sponsors and 15-month-old male and female 5XFAD animals, we implemented contact labeling subcellular proteome of the myelin–axon program.
We found numerous ligand-receptor interactions and dysregulated signaling pathways, including those linked to lipid metabolism, axonal outgrowth, and amyloid-processing.
Expansion microscopy revealed amyloid- aggregation within the internodal periaxonal space and paranodal/juxtaparanodal channels, and it confirmed the subcellular localization of top proteomic hits.
Although overall myelin coverage is preserved, we found altered paranode positioning around amyloid-plaque-associated dystrophic axons, decreased paranode density, and aberrant myelination.
These findings support the hypothesis that the myelin–axon interface is a crucial site for protein aggregation and that AD-related neuro-glial signaling is disrupted.