Summary: A new research reveals that astrocytes, a type of mental body, may transform into muscle tissue through changes in DNA imprinting. Researchers discovered that nerve cells can be produced by astrocytes in particular brain regions because of their special methyl pattern.
A lack of blood offer, such as a stroke, may cause this approach to reprogram astrocytes to create new cells. The results could open the door for novel treatments that maintenance brain injury by promoting the production of muscle cells.
Important Information:
- Through alterations in DNA imprinting, astronomers can turn into muscle cells.
- A strobocytes are triggered by head injuries or strokes to alter and create new neurons.
- This procedure may be a target for upcoming treatments to restore brain damage and treat nerve conditions.
Origin: DKFZ
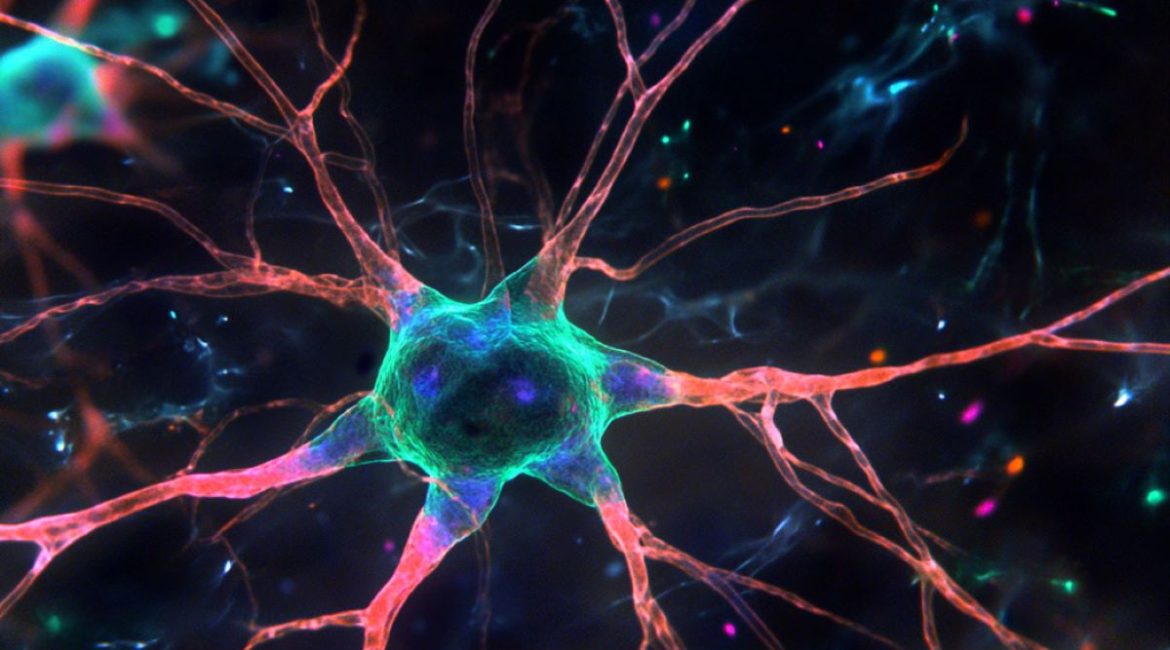
In the mind, many different kinds of tissues interact. In humans, nerve cells ( neurons ) make up less than half of the cells. The remainder are called “glia”. The most common glial cell are astrocytes. They supply the cells with vitamins, form part of the blood-brain challenge, control the connections and help the immune tissue.
However, only a small percentage of astrocytes are capable of producing brain cells, including brain tissue. These unique astrocytes are consequently also referred to as brain plant cells. Mental stem cells and normal astrocytes almost differ in their gene expression, i. electronic. in the exercise of their chromosomes.
Ana Martin-Villalba, a stem cell researcher at the DKFZ, explains how they can do for diverse functions and what constitutes stem cells.
The secret is methyl.
To solve this puzzle, the teams led by Martin-Villalba and Simon Anders ( University of Heidelberg ) isolated both ordinary astrocytes and brain stem cells from one of the regions of the brain where young neurons still develop in adult mice, the “ventricular-subventricular zone” (vSVZ ).
Using mRNA sequencing, the researchers analyzed gene expression at the level of individual cells as well as the methylation patterns ( “methylome” ) throughout the entire genome. They analyzed the imprinting data using a particularly developed tool*.
The term” molecular “markers” used by cells to turn off unused regions of their DNA are DNA imprinting. Methylation is so crucial for determining the cell personality.
The stem cell experts made the observation that mind stem cells have a unique DNA methylation structure that sets them apart from other astrocytes during this research.
In mind stem cell that are otherwise only used by muscle precursor cells are demethylated, unlike regular astrocytes. This enables the brain stem cells to turn on these genes and start producing brain cells themselves, writes Lukas Kremer, the primary author of the most recent release.
Co-first artist Santiago Cerrizuela adds:” This route is denied to ordinary astrocytes, as the required chromosomes are blocked by DNA imprinting”.
A lack of blood supply causes astrocyte reprogramming to plant cell, which in turn encourages new muscle development.
Had methylation also be used to turn astrocytes into mental stem cells in other brain parts, besides the vSVZ? ” This would be an important step for regenerative medicine to repair damaged areas of the brain”, says Ana Martin-Villalba.
Previous research had already demonstrated that a lack of blood offer, as occurs in brain injury or strokes, causes a rise in the number of kid muscle tissue. Do altered methyl characteristics play a role in this process?
The researchers briefly interrupted the plasma supply to the brain of animals in order to check this. As a result, more brain progenitor cells and astrocytes with the standard stem cell methylation profile were discovered as well as a higher number of vSVZ-positive astrocytes.
Our hypothesis is that ordinary astrocytes in healthy brains do not form nerve cells because of their methylation pattern, claims study lead author Martin-Villalba.
Techniques that particularly alter the imprinting profile could be used to create new neurons and address nerve diseases.
” The lack of blood source, in my opinion, causes astrocytes in some brain regions to disperse the ethyl marks on their DNA in a way that makes their stem cell software available. The reprogrammed tissues then begin to distribute and act as precursors for fresh cells, according to Simon Anders.
We might be able to precisely encourage the coming generation of new neurons if we better understand these processes. For instance, after a stroke, we may enhance the body’s self-healing powers, so that the damage may become repaired”.
Why is it necessary to conduct mouse research for this study?
Stroke or other mishaps can cause irreversible brain injury that is frequently severe for the affected person.  , As of today, there is no way to replace lost muscle tissue.  ,
This study seeks to find strategies to promote child mental regeneration.
This calls for a thorough understanding of how and when brain stem cells can become induced to provide a source of fresh muscle tissue. The researchers must examine evolutionary processes that are unique to highly developed mammals in order to accomplish this.  ,
Imaging techniques are not effective for scanning, but each cell’s individual levels require studies to understand genetic reprogramming.  ,
Because the astrocytes ‘ methylation profile changes as soon as they are taken into society, preventing the epigenetic programming can no longer be found, the investigations cannot be conducted on tissues from the lifestyle food.
About this announcement about science and epigenetics research
Author: Sibylle Kohlstädt
Source: DKFZ
Contact: Sibylle Kohlstädt – DKFZ
Image: The image is credited to Neuroscience News
Original Research: The results will appear in Essence