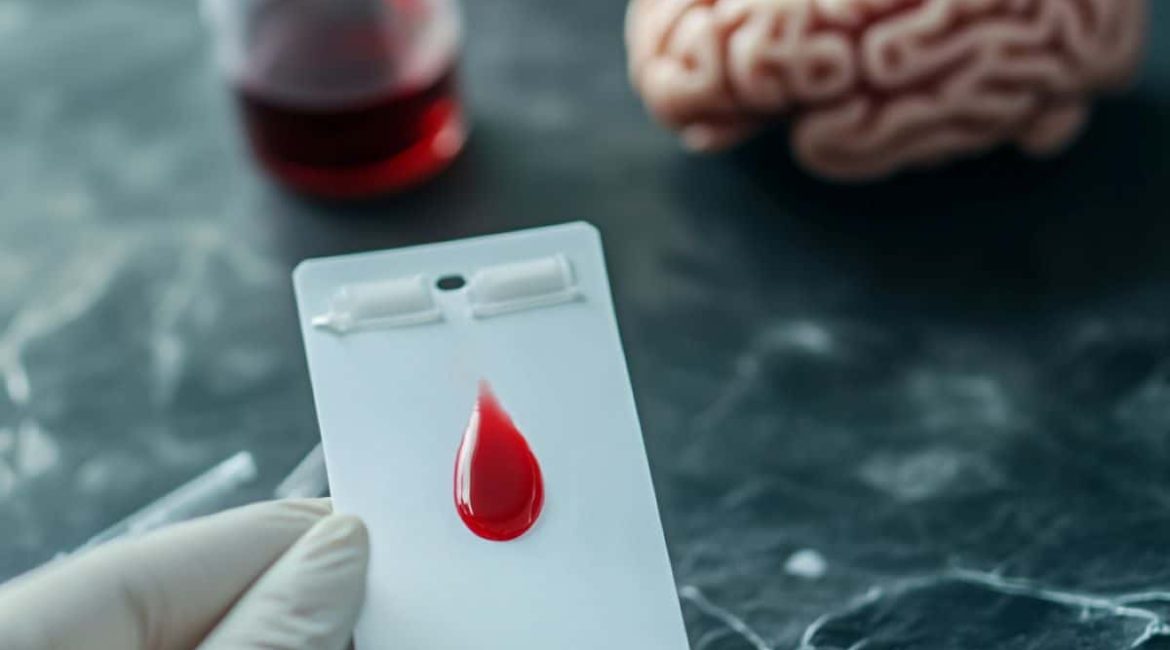
Summary: A novel Alzheimer’s check collects just a few drops of blood from a finger prick, which can be mailed to a lab for analysis. Similar exactness to conventional venous blood sampling has been demonstrated by the test’s ability to measure biomarkers like pTau217.
Unlike conventional methods, this approach does n’t require cold-chain transportation, making it highly accessible for regions with limited infrastructure. With early diagnosis important for treatments like lecanemab, this test could revolutionize Alzheimer’s analysis and research mobility worldwide.
Important Facts:
- The finger-prick check for Alzheimer’s markers is almost as precise as venous sampling.
- Blood tests are mailed to laboratory without requiring specialized vehicles.
- Successful procedures and expanding global research require early diagnosis.
Origin: University of Gothenburgh
A cards that can be delivered in standard message requires a quick hand jerk and a few drops of blood. This approach was quickly generate Alzheimer’s screening much more available worldwide. A German study led by researchers at the University of Gothenburg, Sweden, is paving the way for this process.
The biomarkers used in this exam have been developed over a long period of time and have shown strong performance, first in cerebrospinal fluid, then in vascular blood samples, and finally in hand fluid with cursory vessels.
One or two drops of blood are put on a special card to soon separate blood cells from the blood during the new check. After about 15 minutes, when the cards has dried, it is sent by normal mail to a lab, where modern high-sensitivity methods are used to evaluate it.
As effective as vascular blood sampling
Capillary blood samples from 203 people who took the hand poke test at one of Europe’s five memory centers are included in the latest study. The basic test system was then mailed to the neurochemistry department at the University of Gothenburg, where established indicators for Alzheimer’s, quite as pTau217, were analyzed.
The results were presented at the CTAD ( Clinical Trials on Alzheimer’s Disease ) meeting in Madrid, Spain, on October 30, 2024, by Hanna Huber, a researcher at the University of Gothenburg’s Sahlgrenska Academy:
” The straightforward capillary blood test performs almost as well as capillary samples, but this novel test does not need the transport of dry ice,” according to the author. According to Hanna Huber, this could drastically increase the availability of Alzheimer’s tests in nations and regions with limited access to high-sensitivity assessments.
Within a few years, the exam might be used. Without the need for medical professionals, a new European research is currently being conducted to determine whether the test may be self-administrated, allowing users to prick their own hand and send the test to a lab.
First diagnosis
Lecanemab, a medication that has already been approved in several nations outside of the EU, is a timely development for the test as well as Alzheimer’s treatment. To be effective, these medications require early diagnosis of disease.
The exam opens up opportunities for developing novel studies into Alzheimer’s disease, including its genetic makeup and predominance among people all over the world. The study is not intended to be a general population screening, according to scientists.
The World Health Organization ( WHO ) currently advises against general screening for Alzheimer’s disease, as treatment options have historically been limited, making such screening ethically unsubstantiated.
The research utilizes the blood collection accounts Capitainer®SEP10 and Telimmune.
About this Alzheimer’s disease analysis reports
Author: Margareta Gustafsson Kubista
Source: University of Gothenburg
Contact: Margareta Gustafsson Kubista – University of Gothenburg
Image: The image is credited to Neuroscience News
Original Research: The findings were presented at CTAD ( Clinical Trials on Alzheimer’s Disease )