Summary: Researchers have reprogrammed keyboard cells into embryonic stem tissues using a protein from choanoflagellates, single-celled microorganisms related to animals. This discovery demonstrates that multicellular ancestors had essential genes that enabled the formation of stem cells almost a billion years ago.
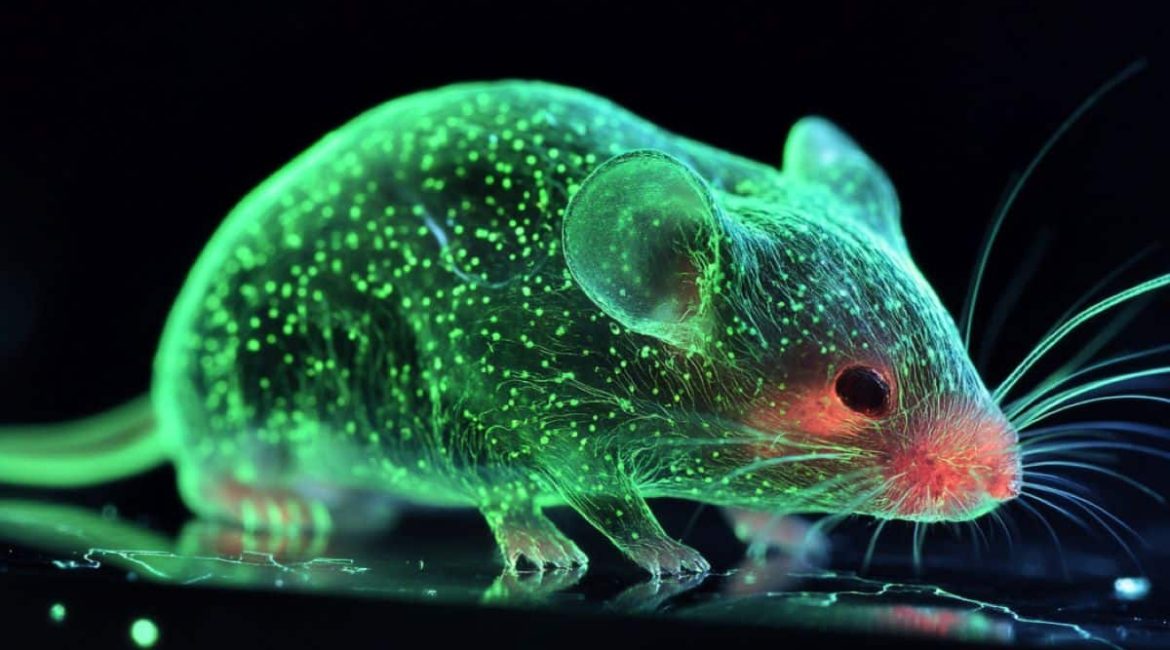
A fantastical mouse was created using the stem cells that developed as examples of how ancient biological tools can be integrated into contemporary mammalian biology. This finding may help to advance regenerative medicine and redefine the roots of stem cells.
Important Facts:
- To convert keyboard Sox2 into stem cells, a choanoflagellate gene was created.
- The altered plant tissues contributed to a fantastical mouse’s development.
- The results suggest plant cell-related genes predate complex species.
Origin: Queen Mary University of London
Published , in , Nature Communications, an international team of researchers has achieved an exceptional step: the development of rat plant cells capable of generating a fully developed mouse using biological tools from a multicellular organism, with which we share a common ancestor that predates animals.
This discovery transforms our understanding of stem cell genetics, giving us a fresh perspective on how the biological connections between species and their prehistoric single-celled family.
Researchers from The University of Hong Kong and Dr. Alex de Mendoza of Queen Mary University of London used a gene from a single-celled species called choanoflagellates to generate stem cells that would later be used to make a living, breathing rat in a science fiction test.  ,
The genomes of cholanomellallates contain versions of the genes Sox and POU, which are known to promote pluripotency, the cellular potential for any cell type, within mammalian stem cells. They are the closest living relatives of animals. A long-held misconception that these genes were unique to animals was challenged by this unexpected discovery.
We’re witnessing an extraordinary continuity of function over nearly a billion years of evolution, according to Dr. de Mendoza, by successfully creating a mouse using molecular tools derived from our single-celled relatives.
The study suggests that important stem cell-related genes may have been created much earlier than stem cells themselves, opening up the door to today’s multicellular life.
The 2012 Nobel prize to Shinya Yamanaka demonstrated that it is possible to obtain stem cells from “differentiated” cells just by expressing four factors, including a Sox ( Sox2 ) and a POU ( Oct4 ) gene.
The University of Hong Kong’s Center for Translational Stem Cell Biology collaborated with Dr. Ralf Jauch’s lab to develop choanoflagellate Sox genes, which enabled the team to reprogramme mouse cells to become pluripotent stem cells using a set of experiments carried out in collaboration with the University of Hong Kong’s Center for Translational Stem Cell Biology. In a developing mouse embryo, these reprogrammed cells were injected to validate their efficacy.
Black fur patches and dark eyes were characteristic of both the donor embryo and the lab-induced stem cells, confirming that these ancient genes played a significant role in making stem cells compatible with the animal’s development.
The research examines how unicellular ancestors used early versions of the Sox and POU proteins, which bind DNA and control other genes, for tasks that would later become essential to the development of animal and stem cells.
” Choanoflagellates , do n’t have stem cells, they’re single-celled organisms, but they have these genes, likely to control basic cellular processes that multicellular animals probably later repurposed for building complex bodies”, explained Dr de Mendoza.
This novel insight emphasizes the versatility of genetic tools in evolution, provides an insight into how early life forms might have used similar mechanisms to promote cellular specialization, long before true multicellular organisms were possible, and addresses the role of recycling in evolution.
Beyond evolutionary biology, this discovery may lead to the development of new regenerative medicine. Scientists may discover new ways to improve stem cell therapies and improve cell reprogramming techniques for treating diseases or recovering damaged tissue by expanding our understanding of how stem cell machinery evolved.
Dr. Jauch praised the advancements that could be made by studying the ancient roots of these genetic tools, noting that experiments with synthetic versions of these genes, which might perform even better than native animal genes in some circumstances, can provide a clearer view of how pluripotency mechanisms can be tweaked or optimized.  ,
About this news about stem cell research and genetics
Author: Ilyana Zolotareva
Source: Queen Mary University of London
Contact: Ilyana Zolotareva – Queen Mary University of London
Image: The image is credited to Neuroscience News
Original Research: Open access.
Alex de Mendoza and colleagues ‘” The emergence of Sox and POU transcription factors predates animal stem cells ‘ ancestry..” Nature Communications
Abstract
The emergence of Sox and POU transcription factors predates animal stem cells ‘ ancestry.
Stem cells are a hallmark of animal multicellularity. Stemness and POU transcription factors are related to stemness, which are regarded as animal innovations and are absent from their unicellular relatives.
We present the POU factors and unicellular sox. Similar to mammalian Sox2, the sox2 and filasterean Sox proteins have DNA-binding specificity. Choanoflagellate—but not filasterean—Sox can replace Sox2 to reprogram mouse somatic cells into induced pluripotent stem cells (iPSCs ) through interacting with the mouse POU member Oct4.
In contrast, choanoflagellate POU has a distinct DNA-binding profile and cannot generate iPSCs. In addition, the loss of Sox2-like properties promoted Sox family subfunctionalization because reconstructed Sox proteins reveal that iPSC formation capacity is prevalent among resurrected sequences.
Our findings suggest that the examination of a set of previously extant transcription factors, where pre-animal Sox was biochemically similar to extant Sox, was a factor for the evolution of animal stem cells, while POU factors necessitated evolutionary changes.